Is your checklist currently looking like this?
- Developed Technology
- Validation
- Publication
- Commercialization
Let abm help you finish your to-do list! With your expertise you can easily validate or publish your work, but let abm’s experts commercialize your technology and share it with thousands of fellow scientists.
Whether you have developed a cell line, virus, protein, or assay design, recoup
the spent research dollars you invested into generating your technology by licensing with
abm! To inquire, please email
us directly at
licensing@abmgood.com or
fill in the form below.
-
 20+ years of experience in establishing mutually beneficial licenses for commercial sale
20+ years of experience in establishing mutually beneficial licenses for commercial sale -
 200,000+ customers and reliable logistics with worldwide distribution in 60+ countries
200,000+ customers and reliable logistics with worldwide distribution in 60+ countries -
 Our facility will be a free biorepository to safely store your technology
Our facility will be a free biorepository to safely store your technology -
 500+ successful commercialization licenses signed to date
500+ successful commercialization licenses signed to date -
 100+ collaborations with renowned institutions and companies worldwide
100+ collaborations with renowned institutions and companies worldwide
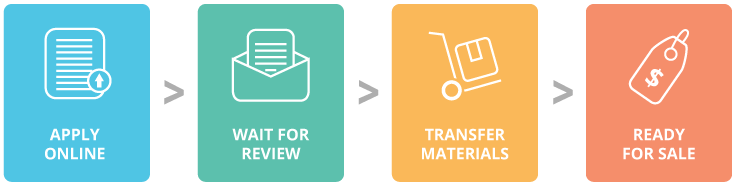
1. Apply online
Let us know about the technology you developed by emailing us at licensing@abmgood.com or filling in the form below.
2. Wait for review
Our Technology Assistants will work with you to review the technology.
3. Transfer materials
Send your materials to abm.Please refer to our protocols for shipping frozen or live cells to abm.
4. Ready for sale
See your technology online ready for sale!
Applied Biological Materials (abm)'s Technology Transfer Office is dedicated to seeking out innovation, protecting abm's intellectual property, and facilitating technology transfers through mutually beneficial licensing terms to bring novel technologies into the market for researcher use.
As true motivators of technology development, our team is ready to connect with principal investigators, fellow technology transfer offices, and liaison offices to facilitate the smooth commercialization of de novo technologies.

The experience has been great. I hope to continue working with you and abm.
Matt Boehm
Mayo Clinic

Angela Trinh, IP Manager
Angela Trinh oversees the daily administration of the technology transfer office, including negotiations, licensing, patent applications, copyright registrations, knowledge transfers, and commercialization strategies.
Email us directly at licensing@abmgood.com or send us your inquiry by completing the form below, and we’ll get in touch shortly.